By Dr. Manal Ghazzawi, Specialist Pharmacist
Many of us were unfamiliar with the names of these large multinational pharmaceutical companies until the COVID-19 pandemic came, and they started manufacturing the wonder vaccines! We have been hearing mainly about the Pfizer-BioNTech (Pfizer) and AstraZeneca (AZ) vaccines with the Pfizer vaccine being the first to receive emergency authorization from the World Health Organisation (WHO) since the outbreak began.
Emergency use of vaccines are authorized by WHO during disease outbreaks, especially those that constitute a public health emergency of international concern, like the COVID-19 pandemic or Ebola virus disease. The following are simple criteria that regulatory agencies across the world follow before any vaccine is authorised to be used by its population:
- Satisfactory manufacturing and facilities
- > = 50% efficacy
- 2 months safety data post vaccination
- End points to include incidence of severe COVID-19
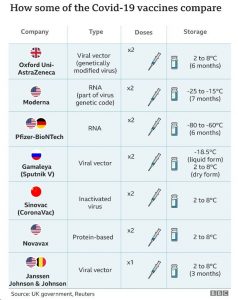
Administration and dosage schedule for approved COVID-19 vaccines
It is recommended that each vaccine series should be completed with the same vaccine brand, except if the first dose of a particular brand was contraindicated. People who have reacted badly to mRNA vaccines (Pfizer-BioNTech and Moderna) should be cautious when taking an Adeno.Cov-2.S vaccine (i.e. AstraZeneca) due to possibility of cross hypersensitivity. Any non-COVID-19 vaccines should not be administered within 14 days of COVID-19 vaccine administration.
For the AstraZeneca (AZ) vaccine, the recommended interval between the two doses a person needs for it to be most effective is 48 days, with the second dose being administered a minimum of four weeks after the first. Age administration is 18 years and older.
For the Pfizer-BioNTech vaccine, 2-dose series are administered 21 days apart. Age indication is 16 years and above.
The Moderna vaccine requires a 2-shot series given 28 days apart and is indicated for individuals aged 18 years and above.
The Johnson and Johnson (J&J) vaccine requires only one dose compared to the previously mentioned three vaccines which need 2 jabs at separate intervals. Age indication for the J&J vaccine is 18 years and above.
The Sinovac vaccine is manufactured in China and, like most traditional vaccines, works by using killed viral particles to expose the body’s immune system to the virus without risking a serious disease response. Only one dose is needed to be administered. Age indication is yet to be confirmed.
As seen in the table below, the Oxford AZ vaccine, Sinovac and J&J vaccines, make perfect sense to be imported into Sierra Leone due to their logistically friendly conditions. In Sierra Leone, we have constraints with cold chain facilities across the country which will make it almost impossible to bring in the other vaccines that have to be stored at freezing temperatures.
Recently, Sierra Leone received 200,000 doses of the Sinovac vaccine donated by the Chinese Government and on 8th March the first batch of 96,000 doses out of expected 528,000 doses of the AZ vaccine arrived.
Vaccine safety and efficacy
The Pfizer and Moderna vaccines have similar efficacy of 95% in preventing symptomatic COVID-19 infection after the second dose administration. For now, we cannot determine with certainty which of the vaccines are the most effective, due to differences in the design of their clinical trials and the different periods of existence of the new COVID-19 variants. These new variants emerged due to a mutation in pre-existing variants, called the ‘E484K mutation’, which has led to the Brazilian, Kent and South African (SA) strains. However, there is a particular concern about the SA variant, which has several changes in the spike protein. The spike protein is what the virus uses to bind itself to infect cells. It is no surprise that SARS COVID-19 mutated as all viruses mutate at an exceptionally increased rate. The COVID-19 virus has mutated a thousand times since its arrival a year ago. These new strains are more recognisable due to the virus’ unique characteristics that make it more able to survive and reproduce.
Clinical trials for both, the Pfizer and Moderna vaccines were conducted before the emergence of the troubling new variants from South African, Kent/Britain and Brazil. In fact, South Africa had to halt its AZ vaccine rollout early February as a result of discovering that the vaccine was not effective against its new variant. Scientists stated that the variant accounts for 90% of new COVID-19 cases in South Africa and it has been identified in 50 other countries so far. A trial of the AZ vaccine which involved about 2,000 individuals found that the vaccine offered “minimal protection” against mild and moderate cases. AZ now intends to develop a third dose that will cover the new variants, while Moderna plans to develop a booster shot after its two dose series. These new strategies involve rigorous technologic processes, therefore reducing production efficiencies and increasing production costs.
The next question that might pop into our minds is if the other approved vaccines are effective against the new variants. The J&J vaccine offered 57% protection against the South African strain and the Novavax vaccine had shown a 60% efficacy in SA which is still fairly good compared to the AZ vaccine. It is important to note that reduced protection does not mean no protection at all.
The mutation that has led to the emergence of the South African variant most likely causes reinfection as it escapes the immune response resulting in a reduction of longevity in neutralizing the antibody response. However, despite the negativities surrounding this predominant South African variant, a very recent study suggests it might offer protection against other variants of the virus. Vaccines that are produced as inactivated viruses such as the Sinovac vaccine, do not mount as enough neutralizing antibodies compared to vaccines that target the spike protein of the virus. The latter effect is produced for example by Pfizer-BioNTech and AZ vaccines.
Concerning the safety of COVID-19 vaccines, large clinical trials have indicated minor side effects, or I would say normal vaccine reactions. These side effects are as a result of the body building protection against the virus, and can include pain at the site of the injection, swollen lymph nodes in the arm where the vaccine was injected, tiredness, headache, muscle or joint aches, nausea, vomiting and fever or chills. The Center for Disease Control and Prevention (CDC) reported 11 cases of anaphylactic reactions (severe allergic reaction) per million doses among people receiving the Pfizer-BioNTech vaccine.
These anaphylactic reactions occur rarely and only among people who are allergic to vaccines generally and can be easily treated with epinephrine a few minutes after vaccination during direct observation. Every drug has its side effects, but there are many misconceptions especially with it being a novel treatment, that might cause immunization hesitancy in people. Misconceptions like the vaccine can cause diseases, infertility or changes your genetic code are incorrect. Vaccine hesitancy could lead to countries not achieving herd immunity and therefore not being able to contain COVID-19 infection, hence the pandemic will continue to linger.
COVID-19 vaccines’ effectiveness in prevention of transmission
From a recent UK preprint study conducted by Pfizer and AZ, their vaccines decreased the number of COVID-19 infections by 86% amongst healthcare workers, after 2 doses of the vaccines had been administered. Also, a pre-published paper in Israel showed that the Pfizer vaccine appeared to reduce all coronavirus infections—including asymptomatic infections—by 89.4% and symptomatic infections by 93.7%. In addition, some evidence supports a 74% drop of asymptomatic infections with J&J vaccines. However, there is need for a lot more time to conduct more peer reviews and larger randomised clinical trials to confirm prevention of transmission by COVID-19 vaccines.
Another critical factor that is worth mentioning is how infectious a vaccinated person is, especially if they develop asymptomatic infection? Again, though it is just a preliminary study, the Pfizer vaccine can help reduce viral load among asymptomatic individuals.
The bottom line is that vaccines which have been approved so far are safe and effective to a degree, but there are still many unanswered questions remaining and stronger evidence needed when it comes to the effectiveness in reducing transmission and high efficacy against the troublesome new variants. However, even minimal protection against symptomatic infections, severe diseases and hospitalization can still make a difference. Kudos to all scientists, public health officials and health care workers around the world who are working day and night to save us all.

Director of Operations
SLURC